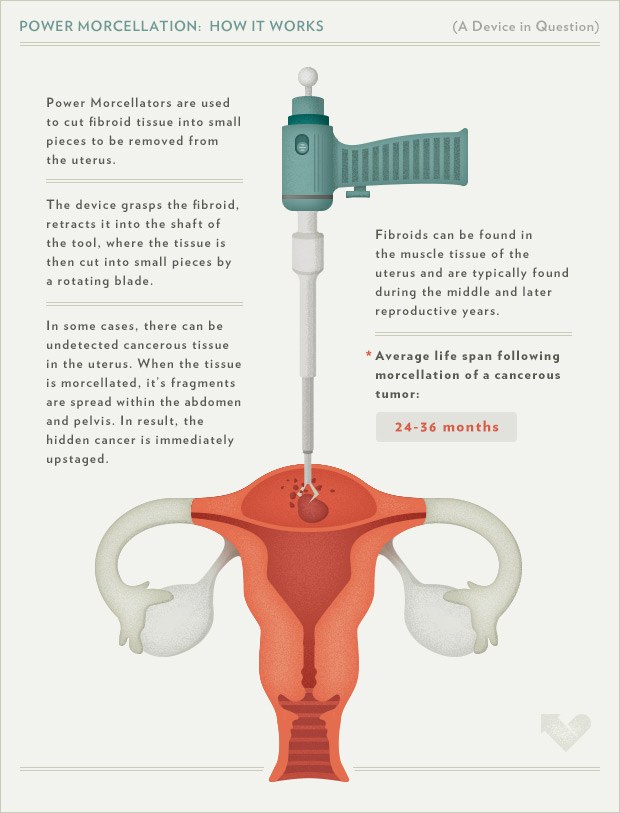
The month of September is both National Ovarian Cancer Awareness and National Gynecological Cancer Awareness Month. This month has come at the perfect time with the women’s cancer community in heated debate over a controversial device called a “Power Morcellator”. Minimally invasive procedures have become more and more common over the years. Before making the decision to undergo either a hysterectomy or myomectomy, make sure to weigh the newly discovered risks of the procedure with the benefits of minimally invasive surgery. For some great information surrounding the ongoing issue, visit American Recall Center’s “Power Morcellator Page“.
Power Morcellator Warning
Power morcellators are medical devices used during various laparoscopic surgeries, such as hysterectomies and myomectomies, that aggressively cut uterine tissue into small pieces. This permits the tissue to be removed through a small incision site, with the proposed benefits including shorter recovery periods, fewer complications, and less pain.
However, due to recent scrutiny and a rise in reports in the medical literature, the FDA issued a safety alert in April 2014 discouraging the use of these devices in uterine and fibroid removal procedures, as they may spread an undetected or unsuspected uterine sarcoma. In this safety alert the FDA also noted there is no reliable method for accurately detecting uterine sarcoma prior to hysterectomy or myomectomy. In addition, according to the FDA, an estimated 1 in 350 women who undergo these procedures may have undiagnosed uterine cancer.
In response to this FDA communication, Johnson & Johnson’s power morcellator manufacturing unit, Ethicon, suspended all worldwide sales and distribution of power morcellators and ordered a voluntary market withdrawal, affecting all Johnson & Johnson morcellator products, including its Gynecare Morcellex, Gynecare X-Tract, and Morcellex Sigma. Several hospitals have suspended use of these devices. At least one insurance carrier will no longer cover procedures involving power morcellation.
To further understand how “Power Morcellation” works, check out the diagram above put together by the American Recall Center.
Power Morcellator Risks and Side Effects
The inherent risk of power morcellation is the dissemination of the broken-up tissue. Benign tissue can become implanted on abdominal structures and organs, resulting in conditions such as fibroids, endometriosis, and adenomyosis, potentially requiring further surgery. Furthermore, power morcellators can spread malignant tissue from an undiagnosed uterine cancer, including leiomyosarcoma, endometrial stromal sarcoma, carcinosarcoma, and endometrial adenocarcinoma, and significantly worsen a patient’s prognosis from treatable to deadly.
To find out more, go to: The Power Morcellator Page
About the American Recall Center

The American Recall Center provides drug & medical device recall information alongside practical healthcare information and support. We aim to build the most comprehensive resource on the Internet for timely & trusted material regarding healthcare topics.
Connect With Them
I, Jamie Tomkins, own and operate TigerStrypes Blog located at https://www.tigerstrypes.com. From time to time you’ll hear about my real life experiences with products and/or services from companies and individuals. Let it be known that I have no affiliation with these said companies, and have not received compensation for reviewing their service/product. The review that I give regarding the product/service is based off my own personal experience, I do not guarantee that your experience will be the same.